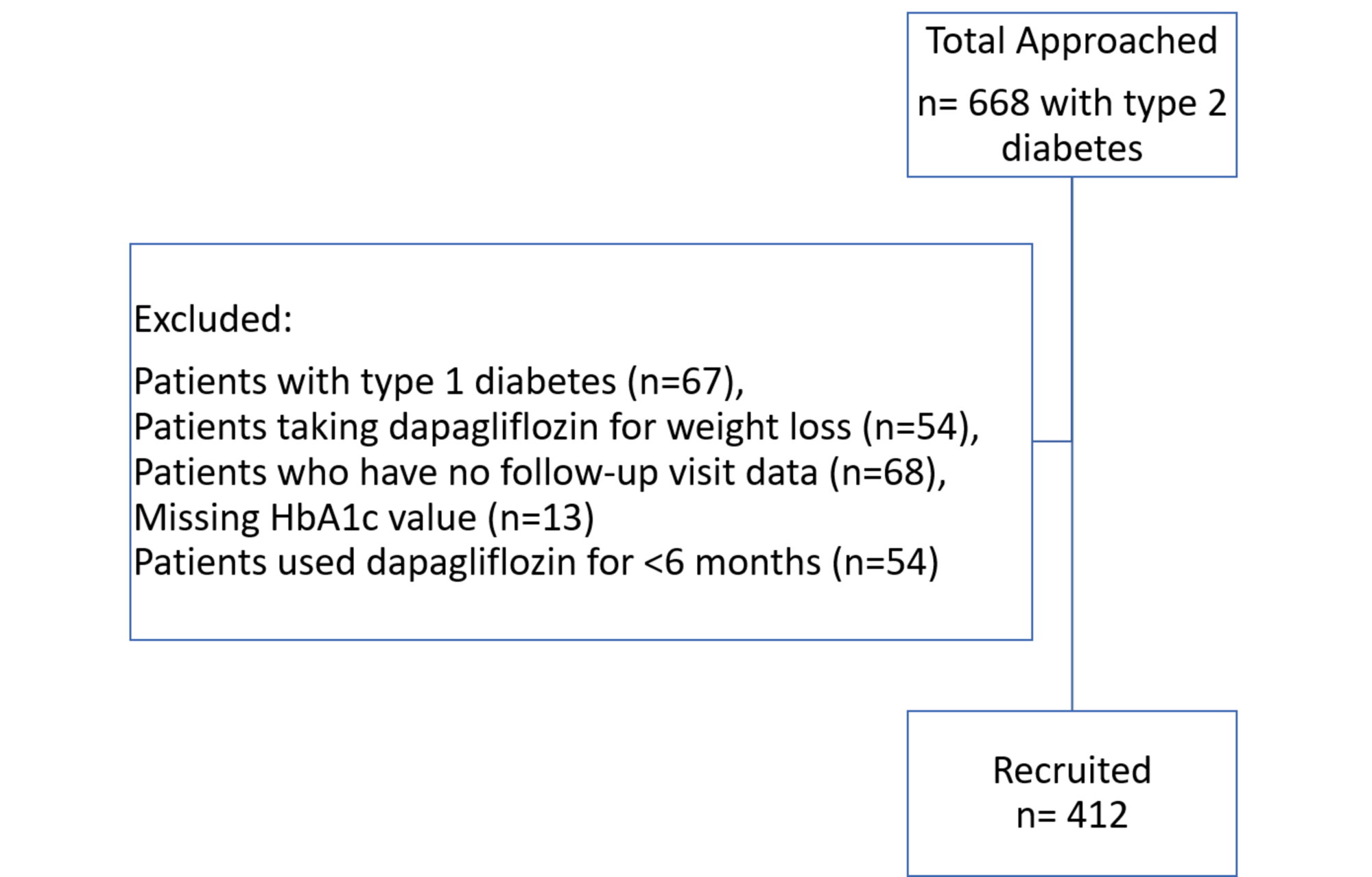
Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study - The Lancet Diabetes & Endocrinology
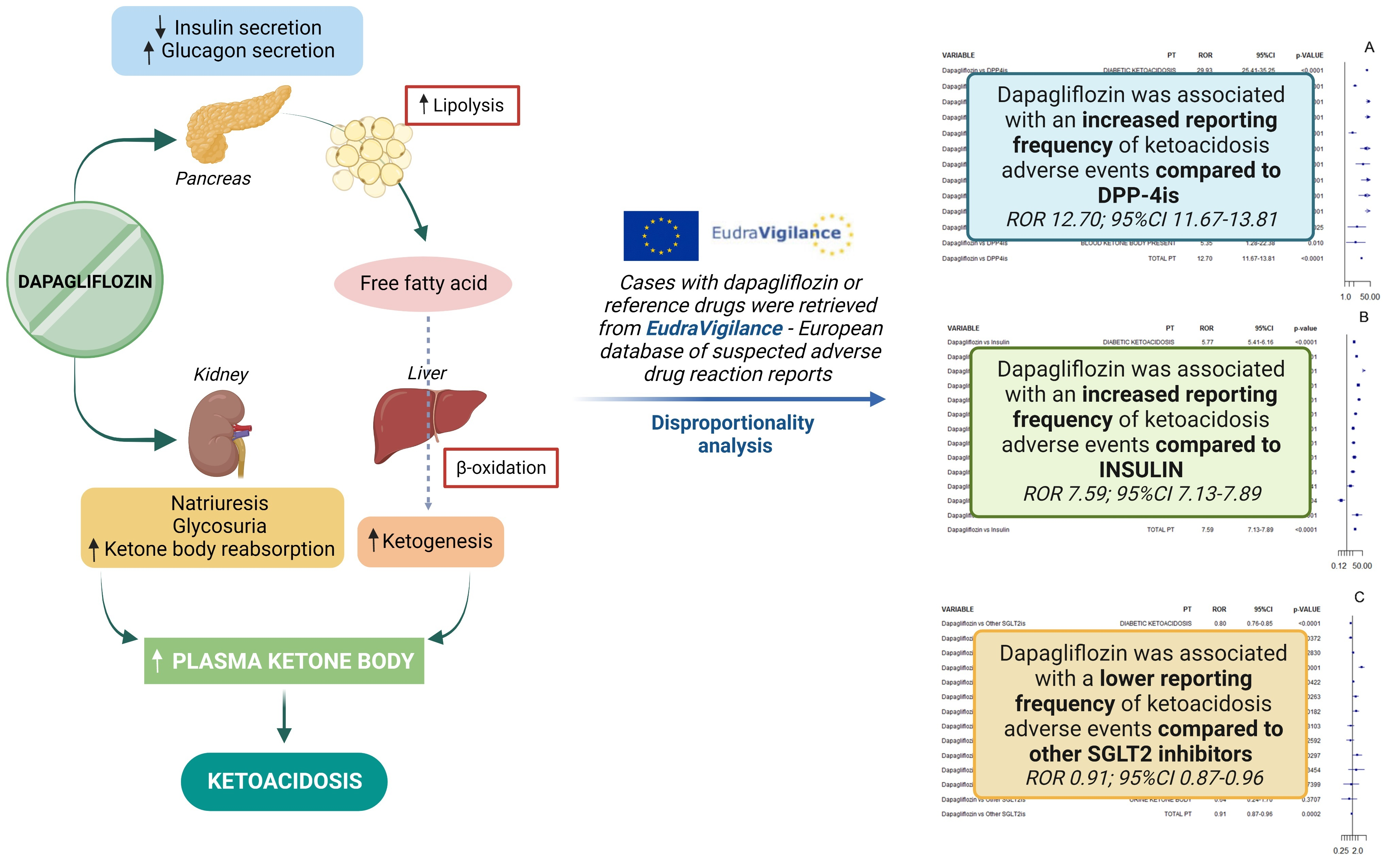
Pharmaceuticals | Free Full-Text | The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study

Non-insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus - The Lancet Diabetes & Endocrinology

A systematic review and dose-response meta-analysis on the efficacy of dapagliflozin in patients with type 1 diabetes mellitus - ScienceDirect